Latest News
The push for greater diversity in clinical trials and what it means for the future of patient recruitment
12 Feb, 20246 MinutesRecruitment problems are the one of the biggest cause of clinical trial delays, with ar...
Recruitment problems are the one of the biggest cause of clinical trial delays, with around 80% of trials failing to meet their enrolment targets. These delays can result in up to $8 million of lost revenue per day for drug development companies. The solution to these delays may lie in the adoption of decentralised clinical trials (DCTs) and non-traditional recruitment methods, but how else will these solutions affect the future of patient recruitment in clinical trials?
Greater diversity among different ethnic groups represented in clinical trials
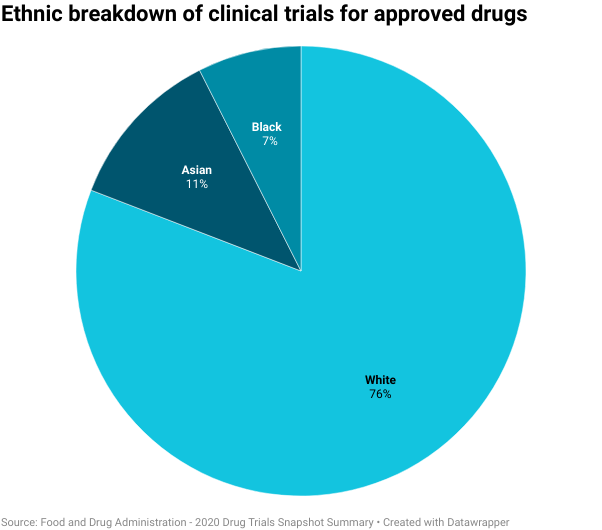
In 2020 the FDA reported that of the 297,000+ participants in clinical trials globally, 75% were white, 11% were Hispanic, and only 7% were Black.
Having this disproportionate patient population means we cannot be 100% sure whether a treatment will work in all populations or what side effects might emerge in different groups. For example, symptoms of conditions such as cancer, diabetes, and heart disease, vary across different ethnic groups, so, having underrepresented demographics in these trials could lead to serious complications further down the line.
However, the future is looking positive. Large organisations are working towards increasing representation of certain racial and ethnic populations in clinical trials.
In 2022 the FDA released a draft guidance document on how to improve enrolment of participants from underrepresented racial and ethnic populations. The draft outlines a proposed plan of action for clinical trial recruiters with the purpose of increasing racial and ethnic diversity.
Improving representation is at the forefront of large organisation’s minds. FDA commissioner Robert M. Califf stated that “Going forward, achieving greater diversity will be a key focus throughout the FDA to facilitate the development of better treatments and better ways to fight diseases that often disproportionately impact diverse communities”.
We’ll also see an increase in gender equality in trials
A 2022 US-based study found that recruitment of women in clinical trials is low, with another study stating that females only account for 29-34% of participants. Being inclusive of only one sex in drug development poses risks for others. For example, in one study it was reported that in over 90% of cases women experienced stronger side effects and experienced adverse drug reactions at nearly double the rate of men, so increasing sex equality in patient recruitment is critical for future clinical trials.
Historically, it has been the case that clinical trial results are generalised between the sexes. So even just having a 29-34% female participant rate could be seen as a huge improvement from where we were 30 years ago. Back in 1993, women were excluded entirely from clinical trials due to concerns surrounding reproduction and also in part due to the unsubstantiated belief that fluctuations in female hormones would make women difficult to study. However, since then there have been higher rates of inclusion of women and this is only going to continue in an upward trajectory.
To continue this progress and increase gender balance clinical trial recruiters will focus on reaching out to female-focused organisations and social media groups while sponsors must incorporate female voices in designing clinical trials to ensure higher participation.
How are we going to see this increase in diversity?
Decentralised trials
Deloitte report that offering DCTs would increase participation across all ethnicities by nearly 20%. A standout figure from their findings was that Asian respondents were 20% more willing to participate in clinical trials if they were offered DCTs. By nature, DCTs encourage patient diversity. They are designed to be remote, so
- Patient populations who previously couldn’t enrol in trials due to location-specific issues now can get involved.
- The dropout rate will be lower. Participants are less likely to have time constraints as they can work the trials around their everyday lives. With 45% of all ethnic minority employees in the UK being given less that a week’s notice for their shifts, in comparison to 28% of white workers, having the option to do it when best suits them should increase uptake.
Another of Deloitte’s reports suggest that biopharma companies are taking underrepresentation seriously with many already leveraging digital solutions and adapting protocol design to enable decentralised trials and better promote both ethnic and gender diversity.
Methods of recruiting patients will change
For clinical trials to reach recruitment targets and encourage a modern patient population to step forward for trials, social media should be used. Just searching “sign up for clinical trials” on Facebook brings up multiple studies for participants to volunteer for. In a 2020 study it was found that targeting potential patients for clinical trials using online methods was an effective recruitment approach and was actually superior in regard to time efficiency and cost-effectiveness in comparison to offline recruitment.
Utilising social media in recruitment efforts offers a different avenue for connecting underrepresented segments of the population with research possibilities. Recruiting patients via social media means that pharma companies aren’t restricted to contacting prospective patients in traditional ways such as through a doctor or specific website. This approach will increase both diversity and enrolment into clinical trials.
With the increase in the uptake of DCTs and the new methods of recruitment via social media, the future of clinical research will be more accessible, more inclusive, and diverse.