Latest News
The move towards decentralised trials and its impact on clinical sector roles
26 Oct, 20227 MinutesDifficulty in reaching target patient populations. High drop-out rates from trial participan...

Difficulty in reaching target patient populations. High drop-out rates from trial participants due to inconvenience. Escalating site costs. These are a few of the existing difficulties of conducting traditional, on-site trials that have spurred the push for digitisation.
But new process brings new remits, and decentralised trials (DCTs) become more commonplace, we could see quite a dramatic shake-up in clinical role requirements that have been standardised for decades.
What changes are we seeing in trials?
Traditionally, for a clinical trial to progress, all those associated with the trial from staff through to patients have had to be present on a trial site. Whilst the tech that enables remote trials to take place has been around for years, the life sciences industry has been slow to embrace it, and firms running trials have been diffident when it comes to disruption.
They can’t be wholly blamed, though – the regulatory hoops a company has to jump through can make trialling new initiatives a huge drain on resource, so the life science industry as a whole has always remained a few steps behind when it comes to digital innovation.
That was until a couple of years ago, when most trials were halted and the ability for healthcare professionals, clinical operations personnel, and patient populations to be present on trial sites was hampered.
For clinical trials to continue in any type of lockdown or societal restriction, a major shakeup needed to be introduced that allowed for patient monitoring, data sharing, and other study protocols to be executed remotely – so the need for decentralised trials was amplified.
It was something that had been in play for about a decade, mainly by major pharma players. In 2021, Pfizer took the first step towards decentralization by conducting a world-first fully ‘virtual’ trial. It allowed patients to use mobile and web-based tech to monitor and input their own trial data without the need for on-site visits or in-person HCP visits. It doesn’t sound ground-breaking from a technical standpoint, but the pilot was only able to take place after the FDA reviewed the initiative and gave it the go-ahead.
Now, telemedicine, digital healthcare interfaces, wearables, and other technology has meant more trials can take place either completely remotely, or in a hybrid remote/on-site capacity – and it’s more than just the major players that are acute to their benefits.
The appeal of taking things off-site
Decentralisation isn’t just a flash in the pan. The knock-on effect of the pandemic might’ve pushed us towards it, but now that we’re out of the other side it doesn’t necessarily mean a return to traditional trial methods.
The benefits of operating a fully or partially remote operation have been explored since that very first Pfizer trial, and unsurprisingly, the patient-centric approach has had a positive impact on clinical outcomes.
Fewer brick-and-mortar trial sites mean there’s less need for patients to travel to and from locations in order to have their progress monitored, so a wider patient population can be reached under more convenient terms. Some of the benefits of decentralisation that are being noted include:
- Reduced trial drop-out rates
- Better patient recruitment efforts
- More diversity in trial participants
- Improved communication between participants and HCPs
- Fewer costs associated with running sites
In a fully remote trial, all aspects of monitoring and data collection happen without the need for in-person contact between the study team and the patient population. Most trial operators with access to tech that enables decentralisation are currently operating a hybrid model rather than a fully decentralised model, so some in-person activity is still required.
Fully decentralised vs hybrid trials
The most widely accepted definition of a fully-decentralised trial is one that takes place from inception to close-out without a central location, trial site, and with no face-to-face interaction between patients and study staff.
All other trials that use some remote processes are considered a hybrid trial, for which there’s no strict definition. This is the more common of the two, and most trials today would fall into this category due to them being tech-enabled in one way or another.

They might use tech to aid communication between monitoring staff and patient populations, or use cloud-enabled data sharing services to pass on trial information between sites, or offer portals or wearable devices for patients to input and monitor their own data remotely – but they also may have a main trial site for operations and logistics that aren’t ready, or suitable, for a fully-remote setup.
Challenges of trial decentralisation
While the benefits pushing sponsors and service providers toward decentralised and hybrid trial models are clear, there are issues that the model raises, and some scepticism about the viability of remote trials across the board. There are still uncertainties in technology, logistics, and approach that need to be overcome before trials are conducted totally off-site.
Adapting to different indications
A decentralised trial naturally lends itself to certain therapy areas over others. Take oncology; although it’s the most studied indication globally, it’s only the fifth most researched therapy area for DCTs.
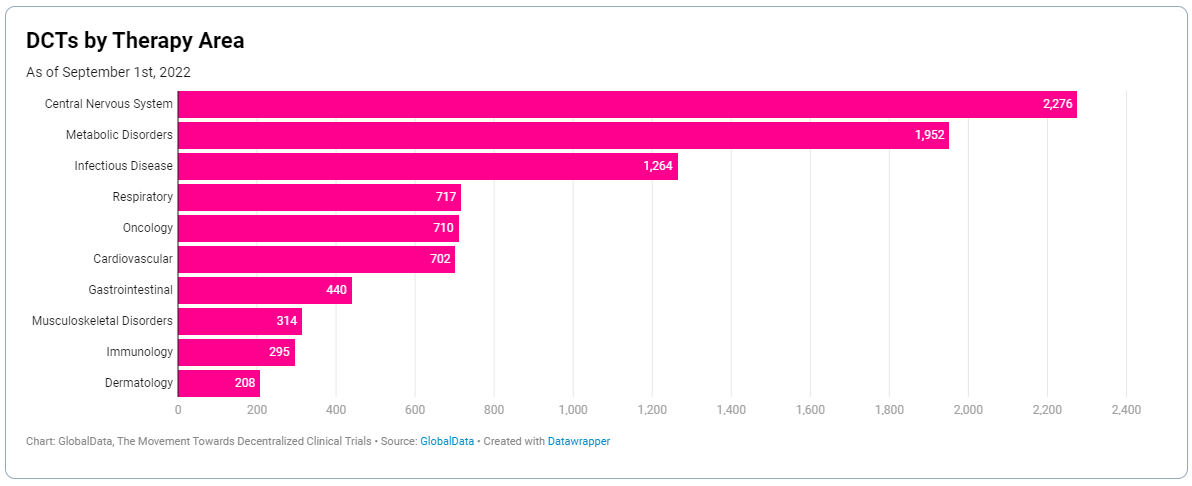
Trial design has to be dictated by the ability to collect quality, usable data from the patient as effectively as possible. This means indications where there’s the potential for data integrity to be lost when conducted remotely, may always need to have an on-site element.
Navigating regulatory barriers
The use of digital technology to facilitate trials has been discussed in guidance outlined by most global healthcare regulators, but remains a relatively new discussion topic. Tech enablement remains an emerging practice, so sponsors can expect existing regulation to change or for new regulation to be introduced.
Trials that take place across multiple geographies also face potential nuances in guidance, so monitoring, consent, home visits, and data integrity will all need to be considered in line with local regulations.
Accessibility
Where the design of DCTs increases accessibility for some patient populations, such as those who are inconvenienced by having to fit visits around their lives, it can negatively impact trial accessibility for others.
People differ in their comfort when it comes to home visits, and some populations are less at ease with some of the technology used to facilitate decentralised trials. This makes data collection tough when it comes to those patient populations, who may be more inclined to take part in a trial in a more traditional, clinical setting.
Internal process changes
When undergoing digital transformation in any setting you’re going to face some common challenges. Technology roll-out, managing scepticism within teams, encouraging widespread adoption, navigating operational changes for the first time – these barriers are faced across the board, and trial design is no different.
A lot of this is cultural, and can be navigated with a strong leadership team with good performance management expertise.
What does this mean for those in clinical trial roles?
Critical review activities that are undertaken by CRAs and other clinical trial specialists over the trial period still must take place in a hybrid or completely decentralised trial. It was something those in the clinical operations space had to adapt to during the pandemic, when global regulatory bodies began accepting remote monitoring and documenting of site activities as valid in a trial setting.
But until now proper infrastructure has been lacking, so going forward we can expect more formalised processes to be put in place to enable those in traditionally on-site trial roles to fully adapt to remote work. This is likely to include resource and support from sponsors to aid the transition to a digital setting as well as retraining on any new processes and systems.
It could prove a selling point to those looking to enter the industry, too, who may not have been drawn to hands-on trial roles due to the fact they are primarily on-site. Work benefits that promote work-life balance, such as flexibility and remote working, can now be offered to those in roles they were previously unsuitable for.